AMSTERDAM — The European Medicines Agency (EMA) and the Heads of Medicines Agencies (HMAs) have jointly released a comprehensive Artificial Intelligence (AI) workplan that extends to 2028, focusing on integrating AI within the European medicines regulatory framework. This strategy aims to leverage AI to enhance personal productivity, automate processes, enrich data insights, and support robust decision-making that benefits public and animal health, according to a press release published on EuropaWire.
The AI workplan, developed by the joint HMA-EMA Big Data Steering Group (BDSG), was formally adopted during EMA’s Management Board meeting in December. It outlines a structured approach to ensure that the European medicines regulatory network (EMRN) remains at the forefront of AI application in medicine regulation.
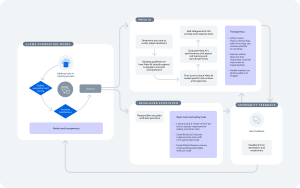
This strategic plan targets four key dimensions: providing continuous guidance, policy, and product support, including a public consultation on the AI reflection paper open until December 2023; developing and utilizing AI tools and technologies with adherence to data protection laws; fostering collaboration and training within the network to keep pace with AI advancements; and promoting experimentation to accelerate learning and innovation across the network.
The workplan not only aims to enhance the efficiency and decision-making capabilities of regulatory processes but also prepares for the upcoming implementation of the EU AI Act in 2024.
The BDSG will regularly update the workplan to incorporate changes in the dynamic landscape of AI technology, including ethical considerations and policy developments. Throughout this process, regulators, medicine developers, academics, patient organizations, and other stakeholders will be actively informed and engaged, ensuring a comprehensive and inclusive approach to AI integration in medicines regulation.
This initiative represents a significant step towards modernizing medicine regulation in Europe and underscores the commitment of EMA and HMAs to harnessing the potential of AI to advance healthcare and regulatory practices.